Short article
The emergence of “omic” sciences in nutrition: a current need
Sandra Perdomo, BSc, PhD,1 Diana Cárdenas, MD, MSc.2
Universidad El Bosque
Summary
In the last decade, the classic approach to nutritional research based on physiological aspects and epidemiological trends has given way to the acquisition of genomic analysis platforms and molecular studies of interaction between nutrients and gene expression.
Nutrigenomics research has focused mainly on the study of chronic diseases such as cancer, obesity, diabetes and cardiovascular disease.They are characterized by presenting dysfunctional biological and nutritional networks, which involve the regulation of multiple genes.
The incorporation of new technologies that allow the application of genetics, genomics and nutrition science.It is a key event to promote the development of research in the nutritional field and to characterize the genetic susceptibility of the population to chronic diseases related to diet as well as the molecular responses to dietary factors.
Keywords: Nutrigenomics, nutrition, genetics (source: MeSH).
Abstract
In the last decade genomic analysis and molecular studies of the interactions among nutrients and of the interactions among processes of gene expression have begun to replace the classical perspective based on physiological and epidemiological aspects of nutrition.
Nutrigenomic research’s primary focus is the study of chronic diseases such as cancer, obesity, diabetes and cardiovascular diseases which are characterized by dysfunctional nutritional and biological pathways regulated by considerable numbers of genes.
The incorporation of new technologies which allow the application of genetics, genomics, and nutritional science is a pivotal event for promoting the development of nutritional research in characterizing genetic susceptibility to development of chronic diseases related to as well as research into molecular responses to dietary factors.
Key words: Nutrigenomics;nutrition;genetics.
Introduction
In the last ten years, research in nutrition has experienced an important change from the epidemiological and physiological approach towards an approach from molecular biology and genetics.
This change in nutritional study is mainly due to the result of three factors that have led to a growing awareness that the effects of nutrition on health and disease cannot be understood without a deep understanding of how nutrients act at the molecular level: first, the results of several genomic projects of large scale have modified nutritional research strategies by highlighting the importance of genes in human nutrition and have provided a wealth of new genetic information to be explored.
Second, There is a growing recognition of the existence of micronutrients and macronutrients that can behave as powerful dietary signals that influence the metabolic programming of cells and have an important role in the control of homeostasis.
Third First, in the field of nutrition, researchers have increasingly begun to recognize that genetic predisposition may be an important factor in the development of major causes of mortality that are linked to diet, such as cardiovascular disease, type II diabetes and cancer.(1, 2)
Traditional nutritional research approach to the sciences Nutrigenomics
In contrast to the traditional nutritional research approach, the sciences nutrigenomics aim to apply the latest technologies to develop a molecular and genetic research approach to nutritional problems.
Due to the complexity of covering the study of the interaction between genes and nutritional factors and the complexity of diet and nutrition, technologies have been developed that are capable of giving us new ideas about the role of food components in the regulation of tissue function and human metabolism and how these processes may be involved or altered during the development of metabolic disorders. .
These technologies are also very sensitive and capable of detecting relatively small deviations from homeostasis and slight effects of dietary factors on the regulation of gene or metabolite levels, for example.
The most recent “omics” tools are potentially capable of meeting these technological requirements.
Theoretical basis of the emergence of Nutrigenomics
Diet is an environmental factor determining nutritional status which plays an important role in the incidence of prevalent chronic diseases.Interindividual genetic variability is a critical determinant of different nutritional requirements.The use of different molecular techniques has allowed the identification of markers of different types (RFLPS restriction fragment length polymorphisms, microsatellite markers, single nucleotide polymorphisms), which are frequently used in the preparation of genomic profiles. and that will allow selecting individuals susceptible to specific diets.(3)
Nutritional process is based on interaction
Essentially, each nutritional process is based in the interaction between a multitude of proteins encoded by a multitude of messenger RNA molecules expressed in a certain type of cell or organ.
Alterations of the levels of messenger RNA and, in turn, of the proteins corresponding parameters (although this does not necessarily always change in parallel) are critical parameters for controlling the flux of a nutrient or metabolite through a biochemical pathway.
Nutrients and non-nutrient components of nutrients Foods can affect gene expression either directly by interference with the gene expression control machinery or by virtue of induced metabolites or metabolic conditions (hormonal status, cellular redox status, etc.) that in turn directly or indirectly alter the genes. messenger RNA levels and/or protein levels.
The application of genomics and proteomics technologies in studies on nutrition, whether in cell cultures, animals or human beings, has the great potential to identify specific markers (biomarkers) that respond to certain nutrients, treatments or a diet.(4)
Molecular studies
New technological tools that have allowed studies to be carried out Increasingly detailed molecular analyzes in the field of nutrition have also helped shift the focus of this area.
Subtle changes in gene expression, even at the single-cell level, can now be measured by quantitative techniques such as real-time PCR and high-density microarray analysis.
The latter allows the nutritional transcriptome to be studied simultaneously, this being one of the important focuses of the field of nutritional genomics or nutrigenomics. (5)
Equally comparable progress in the analysis of nutrition from metabolomic and proteomic perspectives should soon allow the analysis of nutrient responses in biochemical signaling pathways and metabolic function, from gene expression to the physiological condition of individuals (table 1).
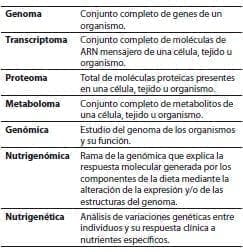
Table 1. Definitions (modified from Gómez Ayala AE.2007).(14)

“Omics” technologies are exploited in human nutritional intervention studies and biological data are used on important mechanisms obtained in different models using specific cell lines as well as biological mouse models.
By combining existing systems biology tools and some in the process of development, the hope is to identify biomarkers and their possible involvement in the most relevant events that develop in the early stages of metabolic disorders.
In this way, information will be obtained on how nutrition can prevent the development of the most prevalent metabolic diseases such as type II diabetes and cardiovascular diseases.
Although each of these analytical platforms, from genomics to proteomics and metabolomics, provide increasingly accurate information that describes a given phenotype, it is the integration of these technologies that provides the optimal means that reveal the effects of a biological agent on an organism;thus, the concept of systems biology (or integrated metabolism).(6)
In fact, an integrated approach to metabolism envisions an attractive and exciting future for the pharmaceutical and food communities, and its search to improve health and prevent disease.
State of the art of “omics” sciences in nutrition
There are currently a large number of technologies that make up the experimental basis of nutritional genomics.
However, the application of this technology is still limited due to the lacking experience in its experimental use and efficient validation that allows its rapid adoption in disciplines where nutritional genomics has a measurable effect such as pharmaceuticals, toxicology, clinical research and pharmaco-genomics.
The greatest challenge of nutritional genomics is in the design of significant studies that apply these methodologies, the development of research capable of deciphering the complex interactions between individual genetic differences, predisposition to diseases and the genomic interaction component in addition to treating to integrate groups that have or are carrying out significant studies in this area.
Diseases that currently reach epidemic proportions, such as cancer, obesity, diabetes and cardiovascular disease, are characterized for presenting dysfunctional biological and nutritional networks, which involve the regulation of multiple genes (polygenic diseases).
The incidence and prevalence rates of these diseases in our country have increased in recent years.Carcinogenesis is a process made up of multiple stages in which both gene expression, protein and metabolite function begin to function aberrantly.
In the post-genomic era, the processes Cells that mediate the initiation of carcinogenesis, including those mediated by dietary factors, have provided important information in our understanding of this disease.
The metabolism observed in tumors is different from that of normal tissues. those that give rise to tumors.
This altered metabolic phenotype allows cancer cells to meet increasing metabolic demands and adapt to environmental changes.
For example, a recent meta-analysis of almost 2,000 microarray studies in which 22 different types of tumors were analyzed. (11) It demonstrated that there is no single common mutation of the genes responsible for the initiation of these tumors, but that instead there are functional signaling pathways including processes shared metabolic processes between various types of cancer.
Thus, dietary intervention to prevent the onset of such diseases is a complex and ambitious goal that requires not only knowledge of how a single food can affect a biological system .But also how a complex mixture (such as diet) of foods can interact to modulate biological functions.(12)
Specific alterations in metabolic pathways can generate opportunities to design new therapeutic approaches.
Future Perspectives
Given emerging evidence that an individual’s dietary requirements may depend of their inherited genes, we can anticipate that important scientific advances will be achieved in understanding the relationships between dietary requirement and genetic background to optimize genome stability;and that the accumulated knowledge about dietary needs for specific genotypes will be used to guide decisions by professionals in this new branch of preventive medicine in what could be called “Genome Health Clinics”.(13)
Conclusions
The integration and application of genetics and genomic technology in nutrition research is necessary to develop research programs in nutrition that are aimed at the prevention and control of chronic diseases through nutritional interventions based on genomics.
Of interest is the integration of relevant computational methods in nutritional genomic research;the improvement of tools applicable to systems biology;and the effective dissemination of information derived from genomics to scientists, policy makers and the community.
In short, new knowledge, produced from the interface of genetics.Genomics and nutrition science are key to promoting the development of research that characterizes genetic susceptibility to diet-related chronic diseases and molecular responses to dietary factors.
Conflict of interest
The authors declare no conflict of interest in the preparation and publication of the article.
Bibliographical references
- 1.Mutch DM, Wahli W, Williamson G. Nutrigenomics and nutrigenetics: the emerging faces of nutrition.Faseb J. 005;19(12): 1602-16.
- 2.Yaktine AL, Pool R. Nutrigenomics and Beyond: Informing the Future-Workshop Summary, NAO Sciences.Editor.Washington, DC: The National Academy Press.2007.
- 3.Garcia-Canas V, Simo C, Leon C, Cifuentes A. Advances in Nutrigenomics research: novel and future analytical approaches to investigate the biological activity of natural compounds and food functions.J Pharm Biomed Anal.2010;51(2): 290-304.
- 4.Minieri M, Di Nardo P. Nutrients: the environmental regulation of cardiovascular gene expression.Nutri Genes2007;2(2): 163-8.
- 5.Bochner BR.New technologies to assess genotype-phenotype relationships.Nat Rev Genet.2003;4(4): 309-14.
- 6.Kaput J. Nutrigenomics research for personalized nutrition and medicine.Curr Opin Biotechnol.2008;19(2): 110-20.Colombian Institute of Family Welfare.National Survey of the Nutritional Situation in Colombia.ENSIN 2010.
- 8.Rodríguez J RF, Peñaloza E, Eslava J, Gómez LC, Sánchez H, Amaya JL, Arenas R, Botiva Y. National Health Survey 2007. National Results.Bogotá: Javeriana Cultural Foundation of Graphic Arts – Javegraf.2009.
- 9.Silveira Rodríguez MB, Martínez-Piñeiro Muñoz and Carraro Casieri R. Nutrigenomics, obesity and public health.Rev Esp Public Health 2007;81(5): 475-87.
Bibliographical Sources
- 10.Wise C, Kaput J. A strategy for analyzing gene-nutrient interactions in type 2 diabetes.J Diabetes Sci Technol.2009;3(4): 710-21.
- 11.Segal E, Friedman N, Koller D, Regev A. A module map showing conditional activity of expression modules in cancer.Nat Genet.2004;36: 1090-8.
- 12.Lamprecht SA, Lipkin M. Chemoprevention of colon cancer by calcium, vitamin D and folate: molecular mechanisms.Nat Rev Cancer.2003;3(8): 601-14.
- 13.Amir RE, Amir O, Paz H, Sagiv M, Mor R, Lewis BS.Genotypephenotype associations between chymase and angiotensin-converting enzyme gene polymorphisms in chronic systolic heart failure patients.Genet Med. 2008;10(8): 593-8.
- 14.Gómez Ayala AE.Nutrigenomics and nutrigenetics.The relationship between food, health and genomics.Offarm.2007;26(4): 78-85.
Authors
1 Sandra Perdomo, BSc, PhD.Bachelor in Science from George Mason University, PhD in Molecular Biology from the University of Salamanca.Coordinator of the Nutrition, Genetics and Metabolism Research Institute, Colombian School of Medicine, El Bosque University, Bogotá, Colombia.
Correspondence: perdomosandra@unbosque.edu.co
2 Diana Cárdenas, MD, MS.Medical University El Bosque, Master of Science and Clinical Nutrition from the University of Paris VII.Director of the Nutrition, Genetics and Metabolism Research Institute, Colombian School of Medicine, El Bosque University, Bogotá, Colombia.
Correspondence: cardenasdiana@unbosque.edu.co
Received: May 2011
Accepted for publication: May 2011
RMNC 2011;2(1): 54-58.
.
