At the beginning of the last century, in 1909, Dr. McArthur used the duodenum as a feeding route in patients with bile fistulas by passing a fine tube through the fistula to the duodenum. ( 10)
However, due to the technical difficulty, risk of infection and permanent fistula, it was decided to quickly abandon this practice.
In 1910, Dr. Max Einhorn built a flexible tube with a metal tip to allow its passage to the duodenum or even the jejunum, which was called the Einhorn tube or duodenal tube and through it the patient was usually fed eggs, milk, butter, sugar and water.
In the same way, he condemned the use of rectal enemas for the administration of nutrients due to the high incidence of rectal irritation and poor absorption of the same.
Once Einhorn introduced his enteral feeding tube (Figure 1), the technique was adopted by highly regarded physicians( 11) and encouraged the development of new nutritional support techniques.
 Figure 1. Einhorn feeding tube.Taken from JAMA 1917;(19): 1395-7.
Figure 1. Einhorn feeding tube.Taken from JAMA 1917;(19): 1395-7.
However, limitations due to feeding intolerance continued to arise due to nutrition boluses in the small intestine and this is how in 1916 , Dr. Jones conceived and developed the use of continuous infusion for feeding, starting with the administration of volumes of 2 ounces to go over a period of two hours to an infusion of 60 to 120 drops per minute and increasing the daily volume by 2 ounces until reach a maximum volume of 12 ounces, which demonstrated greater tolerance, increased survival and allowed feeding for up to a month.(12)
In 1918, Dr. Albert Andresen, from his perspective, understood and introduced the concept of enteral nutrition in the immediate postoperative period, based on the observation of peristaltic movements of the small intestine with total emptying of the jejunum, despite the relative gastroparesis in patients with an open abdomen, during the surgical procedure after a solution of peptonized milk and dextrose.
He then concluded that early jejunal feeding: “is not only safe but is an extremely valuable and recommended procedure.”(13)
In 1939, Dr. David Patton Cuthbertson defined the metabolic response to stress, measuring the urinary secretion of calcium and phosphorus in individuals who had suffered long bone fractures, comparing it with healthy controls.
Injured individuals had greater urinary excretion of phosphorus as well as large urinary losses of nitrogen and potassium, and negative nitrogen balance, which suggested that the amount of this element excreted in urine came from a proteolytic response. generalized striated muscle.(14)
That same year, doctors Stengel and Radvin(15) introduced the concept of adding specific macronutrients through a mixed formula of acidified skim milk, commercial pepsin , sodium bicarbonate, sodium chloride and dextrose, to which they added 1 ml of fish liver oil, 20 mg of thiamine chloride, 50 mg of nicotinic acid and 100 mg of vitamin C.
Stengel and Radvin’s enteral product provided 74 g of protein, 181 g of glucose in 1,024 kcal.(15)
The design of devices and tubes for enteral nutrition continued to increase;In 1940, Abbott and Rawson designed and constructed a sophisticated double-lumen tube, which was passed through the nose and positioned appropriately, such that the proximal lumen was used for gastric decompression and the distal lumen for nutrition (figure 2). (16)

 Figure 2.A, nutrient injection route, A’, solution exit point;B, suction union;B’, point from which the content is aspirated.Taken from JAMA 1939;112: 2414.
Figure 2.A, nutrient injection route, A’, solution exit point;B, suction union;B’, point from which the content is aspirated.Taken from JAMA 1939;112: 2414.
During World War II, doctors in the Soviet Union administered jejunal tube feeding to patients with gunshot wounds to the abdomen using Spasokukotski technique, which consisted of the administration of 400 ml of milk, 50 g of unsalted butter, 2 eggs, 50 g of sugar, 3-5 g of salt and 70 ml of distilled alcohol, in the same operating room. bolus and single dose;and its effects were satisfactory and evident on some occasions, even in the surgery room, with return of color to the cheeks and lips, as well as heat to the body;At the same time, the intestine went from being flaccid and pale to being full, dilated and with peristalsis, in addition to decreasing heart and respiratory rate.(17)
In 1944, the doctor Tui and collaborators achieved a positive nitrogen balance in patients with high calorie consumption and a diet rich in amino acids.In this way, Tui was the first to design the concept of “hypernutrition”, which marked the beginning of the postmodern era of nutrition.(18)
In 1946, Dr. Cecilia Riegel defined that protein requirements should be higher (1.88 g of protein) to achieve positive or at least neutral nitrogen balance in the postoperative period of subtotal gastrectomy or craniectomy in patients with enteral nutrition.(19)
In 1943, Mead Johnson Nutrition introduced the first hydrolyzed protein formula to the market for the treatment of children with allergies and intestinal intolerance.
But in the mid-20th century it began to grow interest in what type of feeding should be used through the tube, and this is how Dr. Pareira, in 1954, jointly developed with Mead Johnson a solution for tube nutrition.
The components of said nutrition were whole milk, non-fat milk powder, calcium caseinate, dextrose, maltodextrose, 8 vitamins (thiamine, riboflavin, ascorbic acid, pantothenate, niacin, pyridoxine, folic acid and vitamin B12), 8 minerals (calcium, phosphorus, potassium, sodium, chlorine, sulfur, magnesium and iron, providing 3,500 kcal, 210 g of protein, 600 g of carbohydrates, 30 g of lipids and vitamins and minerals.
Dr. Pareira, in a large study with 240 patients, successfully administered this solution in continuous infusion or boluses every 4-6 hours and only 7% of them presented gastrointestinal problems.(6)
In the 1950s, Dr. Barron and colleagues at Henry Ford Hospital in Detroit published a series of publications on enteral nutrition, stating that foods prepared in the hospital kitchen were better tolerated and more cost-effective than commercially prepared formulas.(20)
The Henry Ford method for enteral feeding used a polyethylene tube, with a balloon filled with mercury at the end, and through it he delivered baby food, diet from the hospital filtered through a fine mesh and foods processed using machines designed to liquefy solid foods quickly and on a large scale allowing mass production.
The formula supplied 180 g of protein, and 2,600 kcal/ day for a cost of $1.80 per day.
In addition, Barron designed a mechanical pump that allowed a slow and constant flow of the mixture, essential for the success of the technique.(20)
However, two situations were decisive for enteral feeding to be directed towards the development and use of chemical formulas in the 1950s and 1960s: the first, the requirements of essential amino acids in humans. , defined by Rose in 1949;(21) and the second the hospital emphasis on safety, use of aseptic procedures and medical intervention with advanced technology.
In the 1960s and 1970s the rise of Chemical formulas continued to increase and show their benefits.
In 1967, Butler wrote an article praising the use of chemically defined liquid formulas, and raised the need for these formulas to be modified by a nutritionist. according to the needs of each patient.(22)
One of the greatest advances in the 1980s is due to Ponsky who described the technique and experience with percutaneous endoscopic gastrostomy (PEG). (23)
Another giant step was taken by Shike et al., who described the clinical experience with the percutaneous endoscopic jejunostomy technique for patients at increased risk of bronchoaspiration or gastrectomy. previous.(24)
After much controversy, the FDA, in 1995, called a nutrient any supplement that is used to diagnose, cure, mitigate, treat or prevent a disease, defining it as a drug and not as a food.
Present and future
Currently, enteral nutrition is administered to hospitalized patients and at home at an unprecedented rate.The father of modern biochemistry Sir Hans Krebs (1900-1981) said regarding amino acids: “they have multiple functions, but glutamine is the most versatile of all.”
About twenty years ago Dr. Roth found low levels of glutamine in patients with sepsis and related it to their prognosis.(39) In 1988, Dr. Souba managed to determine the role of glutamine as an energy source for the enterocyte and leukocytes. ,( 40) as well as its role in the development of multiple organ failure, by preventing the generation of free radicals and tissue damage.
Since Windmueller’s studies, it is considered essential for the maintenance of intestinal epithelial integrity,(41) the decrease in bacterial translocation and improvement in nitrogen balance.(42)
However, the choice of the enteral route for the administration of glutamine has shown results confusing, and has shown in some studies, a decrease in morbidity and mortality and consequently lower hospital costs in critically ill patients, with better results in groups of patients with hemodynamically stable pressure ulcers, trauma or burns;(43-45) but in patients with non-surgical medical pathology has not shown significant differences,(46-48) so there is controversy about its use and dose.
The parenteral route is preferred over the enteral route, as it shows better results in clinical studies systematically.
Omega-3 essential fatty acids are long-chain fats, discovered in 1929;but its clinical relevance and interest lies in the research of doctors Hans Olaf Bang (deceased) and Jørn Dyerberg in the 1970s and 1980s, who observed a lower development of cardiovascular diseases in natives of Greenland (Inuit or Eskimos), finding a high consumption of omega-3 fatty acids in the population.( 49)
Subsequently, its anti-inflammatory role was determined.They are competitive substrates of arachidonic acid in the cyclooxygenase 2 (COX2) pathway that favor the formation of prostaglandin E3 (PGE3), with anti-inflammatory and immune system-stimulating properties,(50) in addition to inhibiting nuclear factor kappa B, which It reduces the production of proinflammatory cytokines.(51)
Given its potential benefits, it has been used in the hospital setting via the enteral route, in patients with cancer, cardiovascular disease and autoimmune and inflammatory pathologies, within the which we must highlight its use in sepsis(50) and adult respiratory distress syndrome (ARDS).(52)
The beginning of modern science led man to realize not only the need of certain nutrients essential for the maintenance of health, but also of the specific amount of each of them;concepts that allowed us to develop the idea of the influence of nutrients on the onset of different pathologies.
In this sense, the way in which the development and evolution of a disease could be influenced by multiple factors was clarified. , environmental and genetic;the latter were poorly understood until the development of new techniques in molecular biology that allowed the successful capitalization of the human genome sequence in 2001;(53) and the information derived from it has attempted to explain the causes why patients do not respond appropriately. the same way to nutritional support: 1) genetic variability and 2) individual metabolic needs;which has aroused in the last decade a growing interest in the interaction between genes and nutrients as determinants of the prevention, triggering or evolution of various pathologies of each individual.
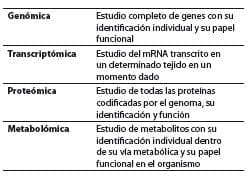
The result of such development has led to the creation of popular terms ending with the Greek suffix omic, meaning “complete” or “whole,” describing the global analysis of genes (genomics), mRNA (transcriptomics), proteins (proteomics), and metabolites (metabolomics), (54-56) words that sometimes lead to a certain type of confusion, so their definition is essential (table 1) to understand the interaction between nutrients, their components and the genome.
Table 1. Terms used in nutritional genomics and their definitions.

In turn, within nutritional genomics There are related but clearly differentiated areas (figure 3): 1) nutrigenomics, which studies the role of nutrients on gene expression and 2) nutrigenetics, defined as the science that studies the effect of genetic variation on the response to diet.(57)
Therefore, both disciplines aim to reveal the nutrient-genome interaction, however, their approaches are different.
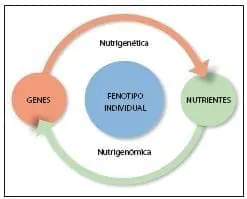
 Figure 3.Adapted from Faseb J. 2005;19(12): 1602-16.(57)
Figure 3.Adapted from Faseb J. 2005;19(12): 1602-16.(57)
Nutrigenomics, a term used for the first time by DellaPena,(58) deals with the effects of nutrients on the transcription of DNA, the translation of mRNA into proteins and the stability of the proteins formed, in addition to the production of different metabolites within cellular metabolic pathways and pathways.(59)
From this In this way, nutrients can be considered signals involved in intracellular communication processes, which in turn affect all the processes involved in cellular function and that together form the cellular, tissue, organic and entire organism phenotype.
Nutrigenetics, a term used for the first time by Dr. Brennan in his book: Nutrigenetics: new concepts for relieving hypoglycemia.(60)
The Nutrigenetics, unlike nutrigenomics, studies the effects of genetic variations on the nutrient-disease relationship and the nutritional requirements for a given individual or population.
This genetic polymorphism is what determines the greatest susceptibility of an individual to suffer from a food-related illness, as well as the clinical response to changes in consumption,(57) and it is from this concept that the term individualized nutrition has been developed.(59)
Another concept of great importance today is epigenetics, a word coined by Conrad Waddington (1942) to describe “the sector of biology that studies the causal interactions between genes and their products that give rise to phenotype”,(61) this concept evolved over four decades, and since the 1990s this term refers to hereditary changes that do not involve changes in the DNA sequence,(62) which is represented by acetylation, methylation and phosphorylation of histones, as well as the chemical modifications of DNA (methylation on the cytosine residues of CpG dinucleotides) that occur throughout life without transformation in the primary base sequence.(63)
Currently, the role of these modifications on the susceptibility and risk of suffering from various diseases has aroused great interest.
Tumor cells are globally hypomethylated in specific regions, this It causes different changes in gene expression and leads to tumor development.Hypomethylation has also been associated with high levels of homocysteine, and therefore, a greater risk of suffering from cardiovascular diseases.
Diet can influence the degree of DNA methylation, in such a way that intake of certain nutrients (folates, choline, vitamin B12 and zinc) can determine a protective factor against the appearance of certain diseases (diabetes, obesity, lupus, schizophrenia and autism, among others).(64-70)
An increasing number of followers recognize foods as direct and indirect regulators of said genetic expression, through various mechanisms (figure 4) that can condition the onset, development, progression and severity of certain pathologies.(71) However, there is still a long way to go before we can extract all the good things that new knowledge in the area of nutritional genomics can give us.

 Figure 4. Fate and role of nutrients in cells.Taken from: Hospital Nutrition 2005;20: 157-64.(71)
Figure 4. Fate and role of nutrients in cells.Taken from: Hospital Nutrition 2005;20: 157-64.(71)
Conclusion
Nutritional support is a standard of care of the hospitalized patient since nutritional deficiency is associated with greater morbidity, mortality and costs, with enteral nutrition being the route of choice in those who maintain a functional gastrointestinal tract.
Resources, technology and techniques of nutritional support have evolved in the last hundred years;New dimensions are discovered in the science of nutrition that allow the development and subspecialization of the disciplines involved in nutritional therapy.
Currently, the first steps are being taken in the promising field of nutritional genomics, which will allow in the near future to increase the effectiveness of nutritional interventions, both in the clinical and population settings, saving time and economic resources and increasing the effectiveness and speed of patient recovery.
In the same way, it will make it possible to identify the different genetic variables in the population, and from them, develop precise nutritional intervention policies in certain risk groups, as well as personalized nutritional treatments that adapt to the genotypic and phenotypic characteristics. of the individual.
Acknowledgments
Many thanks to nurse Sonia Echeverri Serrano, for her educational work in building and strengthening nutritional support in Latin America, which stimulated the preparation of this article.
Conflict of interest
The author declares no conflict of interest.
Funding
The review was carried out with the author’s own resources.
Bibliographical references
- 1.Metabolism, nutrition and shock.4a.edition.Bogotá: Pan American Publishing House 2006. p.1-79.
- 2.Chernoff R. An overview of tube feeding: from ancient times to the future.Nutr Clin Pract.2006;21(4): 408-10.3.Hunter J. A case of paralysis of the muscles of deglutition cured by an artificial mode of conveying food and medicines into the stomach.Trans Soc Improve With Chir Know.1793;1: 182-188.4.Pareira MD.Therapeutic nutrition with tube feeding.Springfield (IL): Charles C. Thomas.1959. p.301-327.
- 5.Cooper SA.Hospital reports.The Lancet.1823;1: 277.
- 6Pareira MD, Conrad EJ, Hicks W, et al.Therapeutic nutrition with tube feeding.J Am Med Assoc.1954;156(9): 810-6.7.Bengmark S, Ortiz J. Enteral nutrition: past and future.Nutr Hosp 2004;21(2): 110-20.8.Brown-Sequard CE.Feeding per rectum in nervous affections.Lancet 1878;1: 144.
- 9Bliss DW.Feeding per rectum: as illustrated in the case of the late President Garfield and others;From “The Medical Record” New York.Seal of the Leland Stanford Junior University 1882. p.1-16.
- 1McArthur L. Some therapeutic possibilities of biliary fistulas.JAMA.1910;54(1): 1-2.11.McWhorter GL.Gastric and duodenal infusion by means of the duodenal tube.JAMA.1917;19: 1395-7.12.Jones CR.Duodenal Feedings.Surg Gynecol Obstetrics.1916;22: 236-40.13.Andresen AF.Immediate jejunal feeding after gastroenterostomy.Ann Surg.1918;67: 565-6.14.Cuthbertson DP, Mcgirr JL, Robertson SM.The effect of bone fracture on the metabolism of the rat, Institute of Physiology, University of Glasgow.1939;29(1): 13-25.15.Stengel A, Ravdin IS.The maintenance of nutrition in surgical patients with a description of the orojejunal method of feeding.Surgery.1939;6: 511-523.16.[ PubMed ] Abbott W, Rawson AJ.A tube for use in postoperative care of gastroenterostomy patients.JAMA.1939;112: 2414.17.Panikov PA.Spasokukotsky’s method of feeding abdominal wounds.Am Rev Soviet Med.1943;1: 32-36.18.Water C, Wright AM, Muholland JH, et al.Studies on surgical convalescence.Ann Surg.1944;120: 99-122.19.Riegel C, Koop CE, Drew J, et al.The nutritional requirements for nitrogen balance in surgical patients during the early postoperative period.J Clin Invest.1947;26(1): 18-23.20.Fallis LS, Barron J. Gastric and jejunal feeding with fine polyethylene tubes.Arch Surg.1952;65(3): 373-1.21.Rose WC.Amino acid requirements of man.Fed Proc.1949;8: 546-52.22.Butler FS.Precision liquid diet helps physically, mentally ill.Top Hosp.1967;45: 60-6
- 2Ponsky JL, Gauderer MWL, Stellato TA.Percutaneous endoscopic gastrostomy.Arch Surg.1983;118: 913-4.24.Shike M, Berner YN, Gerdes H, et al.Percutaneous endoscopic gastrostomy and jejunostomy for long-term feeding in patients with head and neck cancer.Otolaryng Head Neck Surg.1989;101(5): 549-54.25.Mazaki T, Ebisawa K. Enteral versus parenteral nutrition after gastrointestinal surgery: a systematic review and meta-analysis of randomized controlled trials in the English literature.J Gastrointest Surg.2008;12(4): 739-55.26.Enteral vs. parenteral nutrition for the critically ill patient: a combined support should be preferred. Curr Opin Crit Care 2008; 14: 408-14.
- 27. Schloerb PR. Immune-Enhacing diets: Products, components, and their rationales. J Parenter Enteral Nutr. 2001; 25(Suppl 2): S3-S7.
- 28. Cynober LA. Metabolic and Therapeutic Aspects of AminoAcids in Clinical Nutrition. Boca Raton: CRC Press. 2004. p. 595-612.
- 29. Daly JM, Reynolds J, Thom A, Kinsley L, Gallagher MD, Shou J, Ruggieri B. Immune and metabolic effects of arginine in the surgical patient. Ann Surg. 1988; 208: 512-22.
- 30. Freund H, Atamian S, Holroyde J, Fischer JE. Plasma amino acids as predictors of the severity and outcome of sepsis. Ann Surg. 1979; 190: 571-6.
- 31. Luiking YC, Poeze M, Ramsay G, Deutz NE. Reduced citrulline production in sepsis is related to diminished de novo arginine and nitric oxide production. Am J Clin Nutr. 2009; 89: 142-52.
- 32. Ochoa JB, Udekwu AO, Billiar TR, et al. Nitrogen oxide levels in patients after trauma and during sepsis. Ann Surg. 1991; 214: 621-6.
- 33. Bistrian BR. Practical recommendations for immune-enhancing diets. J Nutr 2004; 134(Suppl10): S2868-S72.
- 34. Moore FA, Moore EE, Kudsk KA, Brown RO, Bower RH, Koruda MJ, Baker CC, Barbul A. Clinical benefits of an immune enhancing diet for early posinjury enteral feeding. J Trauma. 1994; 37: 607-615.
- 35. Kudsk KA, Minaard G, Croce MA, Brown RO, Lowrey TS, Pritchard FE, Dickerson RN, Fabian TC. A randomized trial of isonitrogenous enteral diets after severe trauma. An immuneenhancing diet reduces septic complications. Ann Surg. 1996; 224: 531-40.
- 36. Kieft H, Roos AN, van Drunen JD, et al. Clinical outcome of immunonutrition in a heterogeneous intensive care population. Intensive Care Med. 2005; 31: 524-32.
- 37. Tsuei BJ, Bernard AC, Barksdale AR, et al. Supplemental enteral arginine is metabolized to ornithine in injured patients. J Surg Res. 2005; 123: 17-24.
- 38. Chuntrasakul C, Siltham S, Sarasombath S, et al. Comparison of a immunonutrition formula enriched arginine, glutamine and omega-3 fatty acid, with a currently high-enriched enteral nutrition for trauma patients. J Med Assoc Thai. 2003; 86: 552-61.
- 39. Souba W. The gut as a nitrogen processing organ in the metabolic response to critical illness. Nutr Sup Serv. 1988: 15-22.
- 40. Roth E, Funovics J, Mühlbacher F, Schemper M, Mautitz W, Sporn P, Fritsch A. Metabolic disorders in severe abdominal sepsis: glutamine deficiency in skeletal muscle. Clin Nutr. 1982; 1: 25-41.
- 41. Lovat R, Preiser JC. Antioxidant therapy in intensive care. Curr Opin Crit Care. 2003; 9: 266-270.
- 42. Sacks G. Glutamine supplementation in catabolic patients. Ann Pharmacother. 1999; 33: 348-54.
- 43. Houdijk APJ, Rijnsburger ER, Jansen J. Randomized trial of glutamine enriched enteral nutrition on infectious morbidity in patients with multiple trauma. Lancet 1998; 352: 772.
- 44. Jones C, Palmer A, Griffiths R. Randomized Clinical Outcome Study of Critically Ill Patients Given Glutamine Supplemented Enteral Nutrition. Nutrition. 1999; 15: 108-15.
- 45. Zhou YP, Jiang ZM, Sun YH, Wang XR, Ma EL, Wilmore D. The effect of supplemental enteral glutamine on plasma levels, gut function, and outcome in severe burns: a randomized, double-blind, controlled clinical trial. J Parenter Enteral Nutr. 2003; 27: 241-5.
- 46. Hall JC, Dobb G, Hall J, de Sousa R, Brennan L, McCauley R. A prospective randomized trial of enteral glutamine in critical illness.2003; 29: 1710-6.
- 47. Wernerman J. Clinical Use of Glutamine Supplementation. J Nutr. 2008; 138: 2040-4.
- 48. Luo M, Bazargan N, Griffith DP, Estívariz CF, Leader LM, Easley KA, et al. Metabolic effects of enteral versus parenteral alanyl-glutamine dipeptide administration in critically ill patients receiving enteral feeding: a pilot study. Clin Nutr. 2008; 27(2): 297-306.
- 49. Von Schacky C. Prophylaxis of atherosclerosis with marine omega-3 fatty acids. A comprehensive strategy. Ann Intern Med. 1987; 107: 890-899.
- 50. Martin JM, Stapleton RD. Omega-3 fatty acids in critical illness. Nutr Rev. 2010; 68(9): 531-41.
- 51. Lo CJ, Chiu KC, Fu M, et al. Fish oil decreases macrophage tumor necrosis factor gene transcription by altering the NF kappa B activity. J Surg Res. 1999; 82: 216-21.
- 52. Pontes-Arruda A, Demichele S, Seth A, et al. The use of an inflammation- modulating diet in patients with acute lung injury or acute respiratory distress syndrome: a meta-analysis of outcome data. JPEN. J Parenter Enteral Nutr. 2008; 32: 596-605.
- 53. Lander ES, Linton LM, Birren B. Initial sequencing and analysis of the human genome. Nature. 2001; 409: 860-921.
- 54. Young VR. Human Nutrient Requirements: The Challenge of the Post-Genome Era. W.O. Atwater Memorial Lecture and the 2001 ASNS President’s. J Nutr. 2002; 132: 621-9.
- 55. Mooser V, Ordovas JM. ‘Omic’ approaches and lipid metabolism: are these new technologies holding their promises? Curr. Opin. Lipidol. 2003; 14: 115-19.
- 56. Fenech M, El-Sohemy A, Cahill L, Ferguson LR, French TA, Tai ES, et al. Nutrigenetics and nutrigenomics: viewpoints on the current status and applications in nutrition research and practice. J Nutrigenet Nutrigenomics. 2011; 4(2): 69-89.
- 57. Mutch DM, Wahli W, Williamson G. Nutrigenomics and nutrigenetics: the emerging faces of nutrition. FASEB J. 2005; 19(12): 1602-16.
- 58. DellaPenna D. Nutritional genomics: manipulating plant micronutrients to improve human health. Science. 1999; 285(5426): 375-9.
- 59. Ordovas JM, Mooser V. Nutrigenomics and nutrigenetics. Curr Opin Lipidol. 2004; 15(2): 101-8.
- 60. Vaquero P. Genética, nutrición y enfermedad. 1ª edición. Madrid: Editorial Madrid 2008. p. 19-28.
- 61. Goldberg AD, Allis CD, Bernstein E. Epigenetics: a landscape takes shape. Cell. 2007; 128(4): 635-8.
- 62. Haig D. The (dual) origin of epigenetics. Cold Spring Harb Symp Quant Biol. 2004; 69: 67-70.
- 63. Jiang YH, Bressler J, Beaudet AL. Epigenetics and human disease. Annu Rev Genomics Hum Genet. 2004; 5: 479-510.
- 64. Sharma R, Grayson D, Guidotti A, Costa E. Chromatin, DNA methylation and neuron gene regulation the purpose of the package. J Psychiatry Neurosci. 2005; 30(4): 257-263.
- 65. Abdolmaleky HM, Cheng KH, Faraone SV, Wilcox M, Glatt SJ, Gao F, et al. Hypomethylation of MB-COMT promoter is a major risk factor for schizophrenia and bipolar disorder. Hum Mol Genet. 2006; 15(21): 3132-45.
- 66. Melzner I, Scott V, Dorsch K, Fischer P, Wabitsch M, Brüderlein S, et al. Leptin gene expression in human preadipocytes is switched on by maturation-induced demethylation of distinct CpGs in its proximal promoter. J Biol Chem. 2002; 277: 45420-7.
- 67. Jousse C, Parry L, Lambert-Langlais S, Maurin AC, Averous J, Bruhat A, et al. Perinatal undernutrition affects the methylation and expression of the leptin gene in adults: implication for the understanding of metabolic syndrome.2011;25(9):3271-8.68.MacLennan NK , James SJ , Melnyk S , Piroozi A , Jernigan S , Hsu JL , et al.Uteroplacental insufficiency alters DNA methylation, one-carbon metabolism, and histone acetylation in IUGR rats.Physiol Genomics.2004;18(1): 43-50.69.Lu Q, Ray D, Gutsch D, Richardson B. Effect of DNA methylation and chromatin structure on ITGAL expression.Blood.2002;99(12): 4503-8.70.Lu Q, Kaplan M, Ray D, Ray D, Zacharek S, Gutsch D, et al.Demethylation of ITGAL (CD11a) regulatory sequences in systemic lupus erythematosus.Arthritis Rheum.2002;46(5):1282-91.71.Marti A, Moreno-Aliaga MJ, Zulet A, Martinez JA.Advances in molecular nutrition: nutrigenomics and/or nutrigenetics.Nutr Hosp.2005;20: 157-64.
